Annual Report 2019
Preface
The climate problem and various health issues have also brought dynamism and innovation to our field through political and social movements. The course is shifting more and more towards sustainability and innovative production systems, and this is affecting the use of plant protection products and biocidal products.
The coalition government has opted for a sustainable and circular policy. This has resulted in a shift from 'chemistry' to more 'green products', which is reflected in the procedures and assessment methods at both European level for EFSA and ECHA, and at the national level for the Ctgb. The products are also being used differently, and many changes are taking place in the production systems. In accordance with this shift, the permitted use of products must be redefined in conjunction with science-based refinements in the assessment system – for example to prevent resistance from target species.
Public health concerns are also becoming more prominent. Prominent concerns in our field include hormone disruption, neurotoxicity and resistance – such as the resistance of fungi to azoles. This involves various target groups: local residents, consumers, operators and users. Our assessment system is robust, but is also evolving. The increase in data and new developments in science offer opportunities to further refine and optimise our assessment framework and risk assessment. The Ctgb actively participates in this process at the European and national levels.
We are engaging more often in intensive discussions with science and the public. As part of this communication we aim to maintain good contact and relationships not only with applicants, users and operators, but also with citizens, scientists and journalists. Increasingly proactive communication takes many forms: we engage in actual conversations, but also take digital approaches using the website and social media.
These changes and dynamics are driven by innovation: the continued search for new active substances and, increasingly, for green products; innovation in the scope and use of products with precision systems and precision applications, and Integrated Pest Management. Moreover, innovation goes beyond agriculture and horticulture and includes aspects such as rodent control.
The Ctgb made its contribution from a financially sound basis last year.
For their efforts and contributions, the Ctgb owes special thanks to Luuk van Duijn, Eefje den Belder and Walter de Milliano; in 2019 they stepped down from their respective posts as Secretary/Director and Board Member.
Finally, I would like to express my appreciation to our employees, who once again made every effort to ensure that the organisation has continued to develop. We will keep growing, now and in the future, so that we can continue to meet the high expectations of society and politics.
Johan F. de Leeuw, chair
Annual overview: 2019 in a nutshell
Plant protection products and biocidal products may be sold in the Netherlands and Europe only if the producer can demonstrate that they are effective and safe when used as directed. This efficacy and safety is assessed in the Netherlands by the Board for the Authorisation of Plant Protection Products and Biocides (Ctgb), the competent authority in our country for a wide variety of products to control pests and diseases in agriculture and horticulture, products to control rodents, disinfectants for hospitals, swimming pools and cooling towers, but also for anti-fouling paints and products for private citizens. The Ctgb is a semi-autonomous agency. As an independent body in society, it therefore operates in the midst of consumers, industry, agriculture and horticulture, environmental organisations and government ministries and agencies. It assesses plant protection products and biocidal products for safety and efficacy, and then decides whether or not to authorise them. If the Ctgb authorises a product, this means that it is effective when used correctly and is safe for humans, animals and the environment. The Ctgb also provides solicited and unsolicited advice to various ministries.
Decisions and advice
In 2019, the Board made 117 decisions on applications for plant protection products and 69 decisions for biocidal products. The Board rejected five applications for plant protection products and three applications for biocidal products. Multiple authorisations are often issued for a single product due to multiple authorised uses. If the Board authorises a product, this does not mean that all uses requested in the application are also authorised. Some of the requested uses are rejected or are withdrawn by the applicant, due to risks identified by the Ctgb (see figures below)
Uses plant protection products and biocides
| Uses 2019 | |
|---|---|
| PPP granted uses | 77% |
| PPP rejected uses | 23% |
| Biocides granted uses | 94% |
| Biocide rejected uses | 6% |
You can switch on / off individual items in the legend to customise the graphic.
In addition, the instructions for use have often been made more restrictive before approval; this applies to 69% of plant protection products and 33% of biocidal products. This was done so that consumers and growers can be confident that the products they buy and use are safe when used as directed. If one or more standards are exceeded, plant protection products and biocidal products are rejected or their scope of permitted use is restricted.
During the year under review, the Board also advised the Ministry of Agriculture, Nature and Food Quality and the Ministry of Infrastructure and Water Management on 28 exemptions (25 for plant protection products, three for biocidal products).
Authorised products and active substances
The Ctgb reports annually on how many products are authorised in the Netherlands and how many different active substances these products contain. The figures below show that the number of products (and active substances) has increased slightly in the past 10 years. However, these figures provide only limited information on the product assortment per sector (certain sectors have reported a depletion of the product assortment).
Authorised Plant Protection Products and Biocides
| Biocidal products | Plant Protection Products | Active substances PPP | Active substances biocides | |
|---|---|---|---|---|
| 2007 | 759 | 697 | 218 | 81 |
| 2008 | 799 | 699 | 226 | 79 |
| 2009 | 805 | 759 | 232 | 80 |
| 2010 | 829 | 766 | 238 | 83 |
| 2011 | 863 | 771 | 252 | 92 |
| 2012 | 997 | 777 | 256 | 116 |
| 2013 | 1188 | 800 | 257 | 123 |
| 2014 | 1404 | 845 | 262 | 145 |
| 2015 | 1476 | 907 | 271 | 150 |
| 2016 | 1508 | 916 | 279 | 151 |
| 2017 | 1559 | 941 | 277 | 155 |
| 2018 | 1654 | 1000 | 285 | 158 |
| 2019 | 1701 | 1039 | 283 | 158 |
You can switch on / off individual items in the legend to customise the graphic.
Orientation to the outside world
Due to its societal position as an independent body in the midst of consumers, industry, agriculture and horticulture, environmental organisations, government agencies and public bodies, the Ctgb actively communicates with all these groups. This ensures that the assessment system, the decisions and the legal conditions for use remain in line with current practice as much as possible. Scientific assessors and the Board pay working visits to companies, factories and test locations and engage in dialogue with interest groups and NGOs
For example, representatives of the Board visited manufacturers of innovative sprayers to gain an idea of how plant protection products are used in practice: do the instructions on the labels match this use in practice? To gain deeper insights into the use of biocidal products, representatives of the Board visited a factory that fills and packages bottles for soft drinks and other products: how does disinfection of this packaging work in practice? And representatives of the Board also visited a company that provides cleaning and disinfection services for slaughterhouses and other businesses in the food sector. Scientific reviewers visited storage and test locations for potatoes and an experimental farm to gain more insight into efficacy research. They visited a paint manufacturer to understand more about the use of anti-fouling paints and a factory for cleaning and disinfection products to understand more about their manufacture.
In addition, the Ctgb responded to media reports and discussed these reports with experts. In 2019 the Ctgb appeared more often in the media than in previous years, mainly due to the media attention (primarily radio and television) to the authorisation and use of the assessed products.
Local residents
In April, RIVM published a study on the exposure of local residents to pesticides (Onderzoek Bestrijdingsmiddelen en Omwonenden). The study reported finding traces of pesticides in the outdoor air near homes, in dust on the doormats, in house dust and in the urine of adults and children. However, the quantities found remained well below safe standards and the study thus confirmed that in practice the exposure does not exceed the values used by the Ctgb in its risk assessment models. Based on these findings, the Ctgb advised the Minister and the State Secretary.
A television broadcast that same month about the study focused on the concerns of the inhabitants of the Drenthe municipality of Westerveld, where lilies are grown on an increasing number of parcels on which relatively high quantities of crop protection products are used. The heavy spraying equipment also causes traffic nuisance. The residents themselves had also commissioned research into concentrations of plant protection products in their gardens and homes. The amounts they found were well within the permissible exposure standards. From the perspective of the Ctgb's risk assessment, local residents should not be worried. But the residents understandably do not want to find any chemicals in their gardens, even safe levels. Instead of looking at assessment standards for plant protection products, a better solution to the concerns of local residents could be found in improved spatial planning within the municipality. This suggestion is also supported by the Minister of Agriculture, Nature and Food Quality.
The Ctgb entered into discussions with local residents. Several weeks after the broadcast, the Ctgb management and a number of scientific assessors visited the residents in Drenthe to explain the assessment method and to listen to their arguments and concerns. And at the office in Ede, the Ctgb invited critical experts who had participated in the television broadcast to explain their views.
In a similar way, the Ctgb also enters into dialogue with other scientists who come up with new approaches or methodologies. The Ctgb invites them for an explanation, clarification and discussion. Through these discussions, the scientific assessors of the Ctgb can check whether they have taken the current state of science sufficiently into account in their assessments or whether these scientists have valid points that the Ctgb should bring to Europe to improve the assurance of the safe use of plant protection products.
‘Living Laboratory’
In May a video was published online suggesting a big difference between laboratory research into the effects of the neonicotinoid thiacloprid on water fleas (Daphnia) and a study conducted in a ‘living laboratory’. The principle investigator requested the Ctgb to add this study to the European dossier for the reassessment of the substance. After a scientific assessment of the study, the Ctgb replied at the end of May that the European assessment file already contained studies indicating that much stricter standards for thiacloprid are necessary. As a result, the findings of the ‘living laboratory did not yield fundamentally new insights. However, scientific assessors of the Ctgb later visited the ‘living laboratory’ to see whether this research design could add something to the risk assessment of the Ctgb and in Europe. In the autumn, the European Member States decided not to renew the authorisation of thiacloprid.
After a television broadcast in September about the relationship between Parkinson's disease and plant protection products, the Ctgb again published its answers to the programme makers' questions on its website. Distressed viewers and interested parties can then verify the story from the broadcast on the Ctgb website.
The press and the public often respond mainly to the intrinsic properties (toxicity) of a substance; on the website they read how the assessment system works and how legal conditions for use limit exposure and thus keep the risk at a safe level.
Glyphosate
In 2019, Glyphosate was in the news from various perspectives across Europe.
In 2017, the active substance glyphosate was approved for another five years by the European Commission. Based on this approval, the products authorised in the Netherlands that contain glyphosate are currently being reassessed on the basis of all relevant scientific information. In that context, the Ctgb has come to the opinion that one product could no longer be authorised and that another product required strict conditions for the protection of the environment.
Due to the five-year term, the approval of glyphosate must be renewed in 2022. As the dossier for the substance is now very extensive, the European Commission decided that four Member States – France, Sweden, Hungary and the Netherlands – should jointly carry out the reassessment of the substance, so that the European Commission can decide in 2022 whether to renew its approval.
Safety
The Dutch and European assessment and authorisation process is aimed at ensuring the safety of humans, animals and the environment. The assessment frameworks and standards are not static; both continue to develop. Due to new insights, regulations and authorisations are regularly tightened and, if necessary, the Ctgb or Europe withdraws an authorisation. The is often done due to exceedance of the standard for surface water intended for the preparation of drinking water, or due to dangers for pets. (See box.)
In addition, authorisation holders or manufacturers of a product are legally obliged to immediately report new information about potentially harmful or unacceptable effects on humans, animals and the environment to the Ctgb. This applies to plant protection products as well as biocidal products. But third parties can also report new information about the safety of authorised products to the Ctgb. The Ctgb will investigate such a report and assess whether it is necessary to intervene in an authorisation or, for example, to amend the legal conditions for use.
An example of this was a request to withdraw all glyphosate-based products due to human health risks. The Ctgb has explained that it can review an authorisation of a product if there are indications that the authorisation no longer complies with legal requirements. The Ctgb remains alert to any signs that products may not be safe and therefore assessed the studies submitted by this private citizen. However, the studies in question contained a number of scientific uncertainties. As a result, no information could be found in this documentation showing that the authorisations of products with the active substance glyphosate no longer meet the legal requirements.
Requirements
Regulations are in place to protect operators, users and consumers, or they set conditions to ensure efficacy. The exposure time is crucial for the efficacy of disinfectants in, for example, healthcare and the food industry. According to regulations, to kill bacteria you should wash your hands with such a product for between 30 seconds and two minutes. And for surface disinfection, exposure time can be up to an hour. If a user does not adhere to the prescribed exposure time, the Ctgb cannot guarantee efficacy.
Sustainability
In 2019, the Netherlands Environmental Assessment Agency published a midterm evaluation of Dutch plant protection policy: subproject environment. Progress has been made in many areas over the past five years. The water quality has improved and fewer residues of plant protection products are found on food. However, the total use of chemical plant protection products has not yet declined. In society, however, the demand for sustainability is getting stronger and stronger. The Minister of Agriculture, Nature and Fisheries responded to this demand and presented her vision of circular and virtually emission-free agriculture for the future in Agriculture, nature and food: valuable and connected, the Netherlands as a frontrunner in circular agriculture. The Ctgb contributed to this vision document. It has a special Green Team for the assessment of low-risk and ‘green’ products, which makes the Netherlands a frontrunner in these products in Europe. However, ‘green’ products – meaning products of natural origin – are not necessarily without risk. These products must therefore also be assessed for safety. For example, orange peel oil is safe to use in organic cultivation, but it is not a low-risk substance. (See box.)
In 2019, the Ctgb approved various ‘green’ plant protection products with a bacterium as the active substance. This includes a product that works specifically against caterpillars but is not harmful to other insects and a product that prevents fungi from becoming established on plant roots. This bacterium is common in the environment and does not cause disease or pathogenic residues.
In the Netherlands, 30 products have now been authorised on the basis of low-risk substances. In the summer, the European agriculture ministers announced that a total of 75 low-risk products have been authorised in the various EU Member States. At that time, 20 applications were known at the Ctgb for the coming years for the assessment of new substances based on micro-organisms and plant extracts; this is more than 70% of all notified new substances.
The pursuit of sustainability also plays a part in biocidal product authorisations. Through the simplified authorisation procedure, a large biocidal product family was authorised based on the low-risk active substances tartaric acid and sodium benzoate.
Europe
The Ctgb attaches great importance to harmonised legislation and assessment in the European Union. That is why it chose the theme 'The Ctgb in Europe’ for the annual Ctgb client contact day on June 13. The programme included speakers from the European Commission and the European Food Safety Authority (EFSA). They outlined the developments in the assessment frameworks for biocidal products and plant protection products, and industry representatives presented their vision. One of the aims of the programme was to hear relevant points for attention and, if possible, to bring them to the attention of the European Union.
As the Dutch competent authority, the Ctgb operates within the European frameworks of the Plant Protection Products Regulation and the Biocidal Products Regulation. These European regulations have not been worked out in detail. Guidance documents are continuously being developed and specified. The Ctgb actively contributes to this process and works closely with EFSA, the European Chemicals Agency (ECHA) and the competent authorities of the other European Member States; in the Netherlands the Ctgb works closely with various ministries, the Dutch Food and Consumer Product Safety Authority (NVWA), the Human Environment and Transport Inspectorate (ILT) and research institutes such as RIVM and WUR.
During the year under review, the European Commission submitted a proposal to the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) to amend the uniform principles for the assessment of risks for bees. This would implement part of the EFSA 'bee guidance’ from 2013. The proposal was supported by a majority of Member States, including the Netherlands, but was subsequently rejected by the European Parliament. The European Commission also mandated EFSA to review part of the bee guidance. The Ctgb contributed to this revision through two public consultations.
European cooperation is aimed at harmonisation. This means that all Member States of the European Union assess plant protection products and biocidal products in the same way, so that producers, farmers, growers, pest control businesses, hospital hygienists and other users have the same products at their disposal and can compete fairly. However, authorisation will not be exactly the same in all EU countries. Risk mitigation measures in particular cannot be applied in the same way in all countries. Also, not all products and uses are applied for in all Member States, and the dosage may vary. For the risk assessment, however, the Member States apply the same European regulations and guidances to ensure a level playing field.
In recent years, the outcome of the European decision-making process on active substances has become more political and less predictable. As a result, the scientifically substantiated advice from the experts is not always adopted. And the new criteria for endocrine-disrupting substances that applicants now have to take into account has caused additional delays in this decision-making.
Sharing expertise
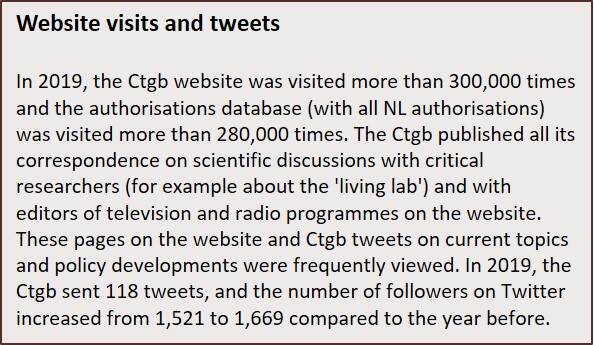
The Ctgb shares the expertise that it acquires in risk assessments and the development of guidances with European forums, applicants, users, other competent authorities and interested parties. Ctgb employees participate in consultations, congresses and conferences. The Ctgb website is a frequently consulted information channel (46% of visitors come from outside the Netherlands), and the Ctgb holds informative meetings and workshops. In 2019, these included workshops on plant protection product efficacy and Tips and Tricks to submit a good BPR application and dossier for biocidal products.
In particular, agricultural journals regularly publish articles about Ctgb decisions, and the Ctgb has good contact with the editorial staffs. The attention for biocidal products is more fragmented, partly due to the multitude of product types. However, in 2019 the Ctgb contributed various articles to biocidal product journals, including an article on the mandatory reporting of harmful effects.
Expertise sharing outside Europe
The Ctgb was again involved in sharing expertise with competent authorities outside Europe, which last year included authorities from Kenya, Jordan and Rwanda. The focus was primarily on biopesticides. Ctgb staff visited Kenya for a WUR-funded project, and the Ctgb provided a two-and-a-half-day programme for a delegation from Jordan. For a delegation from Rwanda, it participated in the ‘exposure visit’ organised by the NVWA with representatives of government, NGOs and industry.
Transparency
The Ctgb is open and transparent about its assessment methodology and the substantiation of its decisions. Authorisation holders, applicants, enforcement bodies, arable farmers, growers and users of disinfectants and other biocidal products can find the information they need on the website and in the authorisations database. The website also provides explanations for the press, the general public, NGOs and consumers. The meetings of the Secretary/Director and chairman with organisations outside the Ctgb are also published online.
After a ruling by the European Court and subsequently by the Trade and Industry Appeals Tribunal, the Ctgb published 85 neonicotinoid studies on its website. After considering all interests, the Ctgb decided to limit confidentiality in this procedure primarily to personal data and the GLP and analysis certificates of laboratories. All other information has been made public.
As it has done with these studies, the Ctgb will publish the results of disclosure requests and Freedom of Information (Wob) requests on its website. A disclaimer points out that these studies were not conducted by the Ctgb, and that the use of a study or its contents without permission of the beneficiary may therefore be prohibited and may be a violation of the rights of the beneficiary. No appeal was brought against this decision, ending an extremely long case. Incidentally, the Ctgb received 10 Freedom of Information Act requests in 2019, about the same as the year before.
Organisation
The Ctgb organisation developed a new multi-year strategy for 2020-2023 and held round-table discussions about this with representatives of ministries, the NVWA, the Dutch Water Authorities; Vewin, sector organisations and NGOs, among others. Based on internal and external analyses and the round table discussion, the Ctgb established the following five priorities for the multi-year programme:
- to carry out our tasks in the chains more effectively, we actively focus on European harmonisation and sustainability,
- to work more effectively and efficiently with stakeholders and colleagues, we embed communication in our policy and aim to enhance this communication,
- to improve our response to changes in the surroundings, we strive to make our organisation more agile,
- we provide our services within statutory or agreed timelines,
- to improve support for our processes and employees, we are developing, integrating and implementing a new application landscape.
Assessing applications
The inflow of applications (in absolute numbers) for crop protection products and biocidal products was lower than expected. Logically, one would expect the Ctgb to be able to take steps to shorten the turnaround times, but the opposite was the case. This is because the applications have become larger and more complex, which the applicants are also aware of. As a result, the amount of work received was not less than expected.
The large bandwidth in size and complexity, combined with the increased unpredictability regarding the moment of submission, made it difficult to coordinate the amount of work and the required capacity (in terms of both hours and expertise). The Ctgb has taken measures to improve the balance between supply and demand. In recent years, a great deal has been invested on the supply side in increasing capacity and providing flexibility to absorb peaks and in the planning methodology. Where possible, work is outsourced externally to Evaluating Bodies, but the available capacity at these organisations is also limited. On the demand side, the Board decided in particular for biocidal products to limit the influx of new applications from Europe, where possible, from 2018.
In line with the expectations expressed in the 2019 work plan, the turnaround times for biocidal products have increased in the past year. For plant protection products, the turnaround times have been reduced slightly. The ambition to realise this for all applications by the end of 2019 – assuming constant circumstances – turned out to be rather optimistic. Applicants have also indicated that the Netherlands is not doing badly compared to other EU Member States and that it must above all work on improving predictability (when will a decision be made?).
In addition to the above factors, the available capacity plays an important role in shortening the turnaround times. There was a slight overcapacity in early 2019. During the year there was both inflow and outflow of employees, which means that a lot of time was invested in training new staff at the expense of productive hours. As a result, the slight overcapacity was no longer available for the rest of the year. Clearing up the backlogs will require some overcapacity, and with the above-mentioned unpredictable inflow and the limited growth capacity of the organisation, it is not possible to achieve this now.
In financial terms, the year closed with a positive operating result and the required accrual of equity was achieved. In 2020, the Ctgb will continue to invest in steps to reduce the amount of work in progress. However, this takes time.
Changing of the guard
After more than six years, Luuk van Duijn stepped down as Secretary/Director in July, and was succeeded by Ingrid Becks. Nicole van Straten was appointed Deputy Secretary/Director. Becks indicated that this will not lead to major shifts in the present course of the Ctgb, which she fully supports. Anita Spooren has been appointed manager of Board Advice & Project Planning.
Risk management
The Ctgb identified five potential business risks for 2019 that could affect the realisation of the work plan: 1) Brexit, 2) the amount of equity (financial buffer), 3) the increased complexity of current and future disclosure requests and objections and appeal cases, 4) capacity and 5) information security.
The Ctgb has taken the following measures to prevent or limit these risks.
Brexit
The Ctgb was prepared to respond flexibly to Brexit if necessary. It also sought timely consultations with the Member States and the European Commission, for example to answer questions about implementation. In collaboration with the Ministries of Agriculture, Nature and Food Quality, the Ctgb provided up-to-date information about the consequences of Brexit for the European assessment and authorisation of products. Brexit became a fact on 31 January 2020, and the work that we are taking over on pending substance and product applications appears to be minor.
Equity capital
The annual structural accrual of equity is progressing well. A modest surcharge in the hourly rate provides for a controlled accrual and, if necessary, for possible financial setbacks.
Capacity
Where possible, the Ctgb anticipated the required capacity in a timely manner by actively recruiting in advance, also taking employee outflow into account. In addition, we focused on maximum internal flexibility so that we can act quickly within and across assessment aspects, during peaks and valleys in work, and can accommodate developments in the range of work on offer. The employee influx is based on controlled growth, i.e. the absorption capacity of the teams is continuously monitored and the timing of the influx and the correct balance between the teams are sufficiently taken into account. Finally, use was made of external Evaluating Bodies where possible.
Information security
The measures that were taken previously also apply in full to 2019. These include strict house rules; security; personal identification and visible pass usage for visitors; a security officer who oversees the security of digitally and physically stored information, connections, applications and hardware. The Ctgb workplaces and infrastructure are secured in accordance with prevailing government standards, and the business applications are strictly separated from our public website. The IT and archive spaces are secured, and an ECHA audit on information security takes place annually. Information security is continuously tightened where necessary in accordance with National Service Information Security Baseline (BIR2017). The information planning architecture (AIP) is providing a reduction of the current application landscape, and investments have been made in new systems and even more secure software and connections.