Registration process
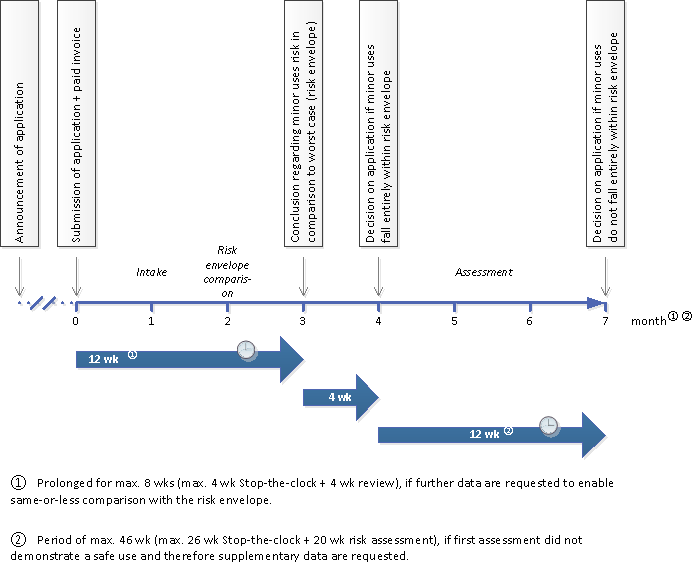
The processing of an application for extension of a national authorisation with minor uses has four phases.
Phase 1. Intake
In the first phase the Ctgb determines whether the application complies with the requirements for completeness or whether more information is required.
The project manager performs an administrative check. In this check it will be determined whether the support for the minor use is sufficient, and if the WG(GA) (Legal Conditions for Use) and the GAP (Good Agricultural Practice) documents will be adopted. The WG(GA) and the GAP documents serve as a basis for further processing. In addition, it is checked whether or not the application is within the scope of the NLKUG. Next, the Ctgb determines if the residue data are adequate, based on whether these data can be extrapolated from the currently authorised uses. The assessors also determine whether a maximum residue limit (MRL) has been established for the minor uses applied for.
Phase 2. Risk envelope comparison
In this phase, the Ctgb considers the risks in the requested extension and compares them with the authorised uses to find out if the risks of minor uses are less or equal compared to those of currently authorised uses. The risk envelope comparison concerns toxicological and environmental risks of :
- the active substance(s)
- the product
- Dosage
- Frequency of use
- Interval of use
- Time of use
- Crop
- Cultivation method (greenhouse, field, storage, etc.)
- Method of use (manual/mechanical; immersion/spraying; upwards, laterally or downwards, and on flower bulbs)
- Spray drift data, including drift percentage
The turnaround for phases 1 and 2 together is 12 weeks. If necessary, the applicant will receive a maximum of four weeks to submit any missing information. After this, the Ctgb has four additional weeks to process this information.
The result of phase 2 can be the following:
- If the extension with minor uses satisfies all conditions and falls entirely within the risk envelope, then the final decision (phase 4) can be made. In that case the total turnaround time is 16 weeks.
- If the extension with minor uses does not satisfy all conditions or does not fall entirely within the risk envelope, then an assessment is required (phases 3 and 4).
Phase 3 risk assessment
In phase 3 the Ctgb assesses the risks for aspects that do not fall within the risk envelop. The risk assessment will be conducted in accordance with the framework at the time of the original assessment or re-registration/renewal (Plant protection products and Biocidal products Regulations (Rgb), Art. 2(2)).
If the supplementary assessment leads to a restriction sentence, this applies only to the extended authorisation under consideration.
The turnaround time for phase 3 can be up to 28 weeks.
During the assessment, it may be necessary to request more specific information from the applicant. The deadline for answering these supplementary questions is 26 weeks. Processing these answers takes 20 weeks and is invoiced separately.
If the Ctgb encounters unacceptable risks for humans, animals or the environment when assessing the extended use, it can reject the application or impose restrictions. The Secretariat presents information about this risk to the Board at an early stage of the process, taking account of the limited crop production area for which the minor use is intended. Based on this information, the Board makes a decision (in principle) on the question on whether this risk is sufficient justification for rejecting the extended authorisation with the minor use.
Phase 4. Preparation of the draft decision and decision making by the Board
Timeline Extension of national product authorisation with minor uses
The amount of time that the Ctgb requires to make a decision on an application for a minor use depends on the nature of the extension with minor uses and the completeness of the dossier.