NLKUG for low-risk products
If the product is approved as a low-risk product in the Netherlands, an NLKUG low-risk (NLKUG-LR) can be requested for it. The NLKUG application form is used and has the same application fee.
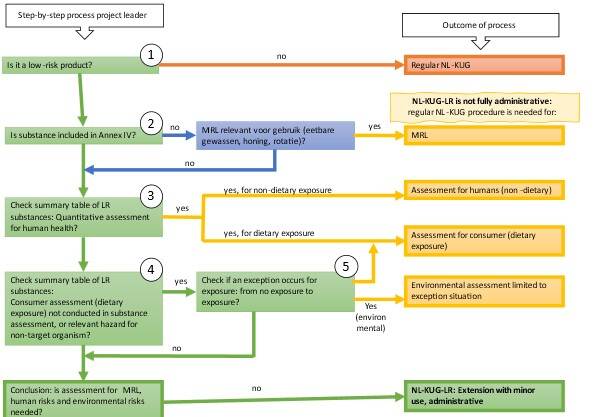
During the intake, the Ctgb considers whether the application can be processed entirely through the administrative route or whether a partial assessment is still required (see table). For example, a partial assessment is required in the following situations:
- if the substance has an MRL;
- if a quantitative assessment of human health effects is required for the low-risk substance;
- if there is no human or environmental exposure from the authorised product/representative use, but there is such exposure for the requested minor uses.